Potassium Chromate And Calcium Chloride
Facts Almost Calcium

Calcium is nature's most renowned structural material. Indeed, calcium is a necessary component of all living things and is also arable in many non-living things, particularly those that assist support life, such equally soil and water. Teeth, sea shells, bones and cave stalactites are all products of calcium.
Interestingly, calcium seems to come up in fifth place wherever it goes: Information technology is the 5th about arable element by mass in the Earth'south crust (later on oxygen, silicon, aluminum and iron); the 5th near abundant dissolved ion in seawater (later on sodium, chloride, magnesium and sulfate); and the fifth nearly arable chemical element in the homo body (afterward oxygen, carbon, hydrogen and nitrogen). It is, withal, the most abundant metallic chemical element in the human body, 99 percent of which tin be constitute in our bones and teeth (about 2 lbs. of it!), according to Chemicool.
In its pure elemental state, calcium is a soft, silvery-white element of group ii. It is important to note, still, that calcium is never found in this isolated country in nature, but exists instead in compounds. Calcium compounds tin be constitute in a variety of minerals, including limestone (calcium carbonate), gypsum (calcium sulfate) and fluorite (calcium fluoride), according to Chemicool. Calcium makes up virtually 4.2 percent of the Globe'due south crust by weight.
In order to isolate pure calcium, information technology must be extracted through electrolysis, a technique that uses a direct electrical current to divide elements from their naturally occurring sources. Once isolated, calcium is quite reactive and volition form a grayish-white oxide and nitride coating when exposed to air.
Calcium (Ca) is No. 20 in the periodic table of the elements, actualization but below magnesium in the aforementioned column (Group IIA) as the other alkali metal world metals (a grouping of metals that are more chemically reactive than most metals). Calcium comes from the Latin discussion "calx," meaning lime. This is not a reference to the fruit, just rather calcium oxide (CaO), the useful edifice material derived from heated limestone.
Discovery
In 1808, Cornish pharmacist and inventor Sir Humphry Davy was the first person to successfully isolate calcium. A few other scientists, Magnus Pontin and Jöns Jacob Berzelius, had come close; they had been able to produce a calcium amalgam after performing electrolysis on a mixture of lime and mercury oxide. This time, Davy repeated their electrolysis method on the same calcium amalgam, but he added more lime to the mixture, producing more of the amalgam from which he was able to distill away the mercury, leaving merely calcium, according to the Majestic Lodge of Chemistry.
Once calcium had been successfully isolated, the element was able to be studied farther, revealing its importance for the survival of all living things.
Just the facts
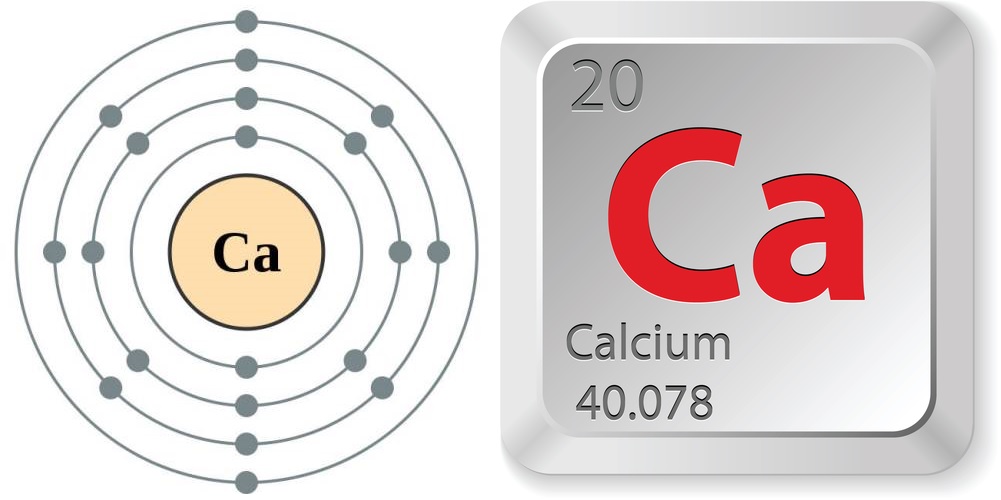
- Diminutive number (number of protons in the nucleus): 20
- Atomic symbol (on the periodic table of the elements): Ca
- Atomic weight (average mass of the atom): 40.078
- Density: 1.55 grams per cubic centimeter
- Phase at room temperature: solid
- Melting point: i,548 degrees Fahrenheit (842 degrees Celsius)
- Boiling bespeak: ii,703 F (1,484 C)
- Number of isotopes (atoms of the aforementioned element with a different number of neutrons): 24; 5 stable
- Virtually common isotopes: Ca-twoscore (97 percent of natural abundance); Ca-44 (two pct of natural abundance); Ca-42 (0.6 percent of natural abundance); Ca-48 (0.2 percent of natural affluence); Ca-43 (0.i percent of natural abundance); Ca-46 (0.004 percent of natural affluence.
Essential mineral
Calcium is extremely important to the human body. Not only is it vital for bones and teeth, but information technology assists in muscle movement by carrying messages from the brain to all our trunk parts. Cells in all living things must communicate with, or "point," one another. Calcium ions human activity equally vital messengers between these cells and are necessary in all multicellular life forms. They also assist in the release of hormones and enzymes.
In food, calcium is found in mineral course. Foods high in calcium include dairy products — such every bit milk, cheese and yogurt — and some vegetables, such equally kale, watercress, spinach and broccoli. In lodge for calcium to absorb properly, information technology should exist taken with vitamin D. Magnesium is as well necessary for proper assimilation and employ of calcium in the trunk. In fact, if nosotros take too much calcium and not plenty magnesium, it tin can crusade issues in the body.
Who knew?
- Lime, or calcium oxide, produces a brilliant, intense low-cal when exposed to an oxyhydrogen flame, co-ordinate to Chemicool. In the 1800s, before electricity was invented, this substance was used to light up the theater and so actors truly performed "in the limelight" — hence the saying.
- When turtles (typically pet turtles) don't have plenty calcium circulating through their blood, they may get an ailment chosen Metabolic Bone Illness, normally known as Soft Vanquish Syndrome. For a turtle to be good for you, the calcium to phosphorous ratio should exist 2:1. When their calcium levels are low, however, the mineral is leached from their bones in the body's attempt to balance things out. The result is soft bones, weakness and a soft, deformed vanquish, and it often results in death. The disease tin can be prevented with a proper nutrition and adequate sunlight (or other appropriate lighting for reptiles).
- Stalactites and stalagmites, the icicle-shaped formations institute in hugger-mugger caverns, are formed slowly over fourth dimension by the build-up of calcite residue. This occurs when water seeps through the cracks in the ceiling of a limestone cave, dissolving and carrying forth traces of calcite, the building material of limestone. Every bit the water drips from the ceiling, this calcite residue begins to build up at the site of the drip, eventually resulting in icicle-shaped stalactites hanging from the cave ceiling. This water dripping from the stalactites so forms stalagmites on the footing below.
- Many nutritionists recommend a calcium-magnesium ratio of ii:1. Only although our bodies require more calcium, we are really more likely to become deficient in magnesium. This is because our bodies tend to store and recycle calcium, while magnesium gets used or excreted and must be replenished on a daily basis.
- Calcium carbonate is the active ingredient in many antacids, such equally Tums and Rolaids. The alkaline compound works by neutralizing the stomach acid responsible for heartburn and indigestion.
Good for you soil
Not only is calcium essential for human life, just it is as well an essential food for plant growth. In near types of soils, calcium is made available to plants through the weathering of minerals. As an alkaline earth metallic, calcium plays a vital part in controlling soil pH (potential of hydrogen), a measure of the soil's acidity or alkalinity.
The availability of calcium tin can indirectly touch many microbial processes that are sensitive to soil pH, such as decomposition, nitrogen mineralization and nitrification, said Feike A. Dijkstra, a biogeochemist and associate professor at the Middle for Carbon, Water and Food at the University of Sydney.
Calcium is typically arable in almost soils with some areas having naturally college or lower levels of calcium. "High levels of calcium in the soil are ordinarily found in arid and semi-arid regions where potential evapotranspiration is larger than actual rainfall," Dijkstra told Live Science. "Certain plants accept adjusted to calcium-rich and alkaline soils, and are chosen calcicoles. In contrast, calcifuges are plants that thrive in calcium-poor and acidic soils."
Issues can occur when there is a pH imbalance in the soil. Soil with an glut of calcium can result in the pH being too loftier (higher up 7), or alkali metal, and this sometimes "reduces the solubility of nutrients such as phosphate and many micro-nutrients, which can and then limit plant growth," Dijkstra explained. The bigger problem, nevertheless, typically lies in soil with also little calcium, as this results in soil acidification. This can happen when calcium is leached out of the soil through heavy rain or even more problematic — acrid rain.
"During the 1970s and 1980s acrid pelting was a major problem affecting many forests in northeastern America and Europe," Dijkstra said. "When acid rain reaches the soil, the protons of these strong acids in acid rain supplant calcium cations on the substitution sites, and the calcium is leached out of the soil profile."
"Acid rain killed many forests because the associated soil acidification resulted in increased solubility of aluminum. Aluminum is toxic to plants when it gets above certain concentrations in the soil," he said. "Since the Acrid Rain Program established under the 1990 Clean Air Act, acrid pelting has become less of a problem for forests in the northeastern U.Southward."
Uses
Calcium compounds accept a wide multifariousness of uses, particularly in the making of construction materials. Gypsum, or calcium sulfate, is used in making plaster and likewise "plaster of Paris," a heavy white powder that, when mixed with water, hardens into a cast to set fractured bones.
Limestone, or calcium carbonate, is used directly as structure textile and indirectly for cement. When limestone is heated it releases carbon dioxide, leaving backside quicklime (calcium oxide). When quicklime is mixed with water, information technology creates slaked lime (calcium hydroxide) which is used to make cement. Slaked lime is also used equally a soil conditioner and as a water treatment amanuensis to reduce acidity. When mixed with sand, slaked lime pulls in carbon dioxide from the air and hardens into lime plaster, according to the Royal Guild of Chemical science.
Pure calcium metallic is used as a reducing amanuensis in the preparation of other types of metals, such as thorium and uranium and zirconium. It tin can too exist used as an alloying agent for aluminum, copper, lead and magnesium alloys, or as a deoxidizer, desulfurizer and decarburizer for a variety of ferrous and nonferrous alloys.
Additional resources
- Jefferson Lab: The Element Calcium
- Los Alamos National Lab: Calcium
- Royal Society of Chemistry: Calcium
Potassium Chromate And Calcium Chloride,
Source: https://www.livescience.com/29070-calcium.html
Posted by: strangefaleas.blogspot.com


0 Response to "Potassium Chromate And Calcium Chloride"
Post a Comment